Abstract
Introduction: Momelotinib (MMB), an oral ACVR1, JAK1 and JAK2 inhibitor, has previously demonstrated clinical activity on symptoms, anemia and spleen volume in JAK inhibitor (JAKi)-naïve and JAKi -experienced patients with myelofibrosis (MF). A commonly used anemia endpoint, transfusion independence response (TI-R), is defined as the absence of transfusions and no hemoglobin (Hgb) measurements below 8 g/dL during the 12 weeks preceding Week 24 (W24). As previously reported from the SIMPLIFY-1 Phase 3 study of JAKi-naïve patients with MF, patients receiving MMB who achieved TI-R experienced prolonged overall survival (OS) compared to those who were non-TI-R. Hence, TI-R can provide a surrogate for long-term clinical benefit (Mesa et al. Leukemia. 2022).
The TI-R endpoint, however, does not fully describe transfusion burden outcomes for patients not meeting the definition of TI-R. Dynamic and time-to-event analyses have previously demonstrated MMB can reduce overall transfusion burden compared to ruxolitinib (RUX) in JAKi-naïve patients including in those who do not achieve TI-R (Mesa et al. Leuk Lymphoma. 2022). To better understand the impact of MMB on TI-R, OS and transfusion burden in JAKi-experienced patients, similar analyses were applied to the ongoing Phase 3 MOMENTUM study which is investigating MMB vs danazol (DAN) in anemic, symptomatic patients with MF who have previously been treated with a JAKi.
Methods: MOMENTUM is a double-blind, randomized (2:1) study of MMB 200 mg daily (130 patients) vs DAN 600 mg daily (65 patients) with a randomized treatment (RT) period of 24 weeks, after which patients could receive open-label (OL) MMB. Eligible patients had primary or post-ET/PV MF; DIPSS high risk, Int-2, or Int-1 MF; MFSAF total symptom score ≥10; Hgb <10 g/dL; prior JAKi for ≥90 days or ≥28 days with RBC transfusions ≥4 units in 8 weeks or Grade 3/4 thrombocytopenia, anemia, or hematoma; and palpable spleen ≥5 cm. Anemia benefit was evaluated by TI-R, Kaplan-Meier (KM) estimates of the proportion of patients who required zero units transfused during RT, hazard ratio (HR) of RBC units transfused as recurrent events, HR of time to 1st, 3rd and 5th transfusion unit and odds ratio (OR) of having zero units transfused estimated by a zero-inflated negative binomial model. Survival was estimated using KM analysis. Transfusion dependence (TD) was defined as requiring ≥4 units of RBC transfusions in the 8 weeks immediately prior with each transfusion in response to a Hgb assessment of ≤9.5 g/dL. Transfusion requiring (TR) was defined as neither TD nor TI.
Results: Mean duration of prior JAKi therapy was 139 weeks in the MMB arm and 125 weeks in the DAN arm. The study population was highly anemic with a mean Hgb of 8.1 g/dL in the MMB arm and 7.9 g/dL in the DAN arm; 48% and 49% of MMB and DAN patients respectively had baseline Hgb <8 g/dL. At baseline, 13% and 15% of MMB and DAN patients were TI, respectively, whereas a majority (49% MMB; 52% DAN) were TD.
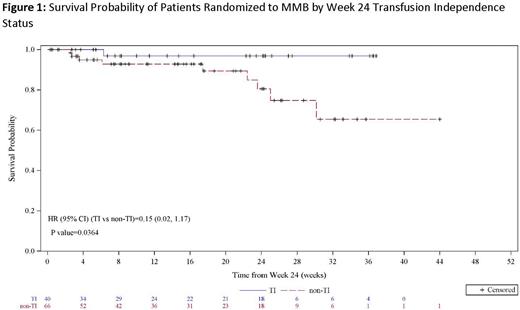
The rate of W24 TI-R was 31% and 20% for the MMB and DAN arms, respectively (non-inferiority p=0.0064). Over the 24-week RT period, 35% of MMB patients had zero units transfused compared to 17% of DAN patients (OR=2.7; p=0.0107). Regardless of baseline transfusion status (TD, TI, or TR), patients randomized to MMB received fewer mean cumulative RBC units during RT compared to those randomized to DAN. Time-to-event-analyses showed median days to 1st, 3rd, and 5th transfusion on MMB was >2 times that on DAN (MMB:40, 106 and not reached; DAN:15, 52, 109). Of the 63 MMB patients with baseline TD, 9 (14%) were TI at the end of W24 and 19 (30%) were TR. Of the 34 DAN patients with baseline TD, 9% were TI at the end of W24 and 9% were TR. Interim OS analysis of both study arms shows that patients who achieve W24 TI-R have prolonged OS compared to those who were non-TI-R; MMB arm shown in Figure 1 (HR=0.15; p=0.0364).
Discussion: Anemia is a major unmet need in MF that is associated with poor prognosis and frequently managed with recurrent transfusions, which are costly in terms of reduced quality of life and OS as well as increased healthcare resource utilization. The analyses presented herein demonstrate that patients receiving MMB benefitted from increased likelihood of becoming TI and lower transfusion burden compared with patients receiving DAN. These findings are consistent with previously reported data from the SIMPLIFY studies of MMB and suggest that TI-R at W24 is a potential surrogate for improved OS.
Disclosures
Verstovsek:Roche: Research Funding; Gilead: Research Funding; Novartis: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; CTI BioPharma Corp.: Research Funding; ItalPharma: Research Funding; NS Pharma: Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation Pharmaceuticals: Consultancy; PharmaEssentia: Research Funding; Genentech: Research Funding; Promedior: Research Funding; Protagonist Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy. Oh:PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Disc Medicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgne/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kiladjian:AOP Orphan: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Platzbecker:Silence Therapeutics: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Geron: Honoraria; Abbvie: Honoraria; Jazz: Honoraria; BMS/Celgene: Honoraria; Janssen: Honoraria. Passamonti:BMS: Research Funding; Novartis, Celgene, BMS, Abbvie, Janssen, Roche, AOP Orphan, Karyopharma, KYOWA KIRIN, Mei: Consultancy, Honoraria, Speakers Bureau. Gerds:Kratos Pharmaceuticals: Research Funding; Accurate Pharmaceuticals: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago BioSciences: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Vannucchi:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AOP Orphans Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Blueprint: Membership on an entity's Board of Directors or advisory committees, Other: NA; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Other: NA; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees. McLornan:NOVARTIS: Honoraria, Research Funding, Speakers Bureau; CELGENE BMS: Research Funding, Speakers Bureau; ABBVIE: Speakers Bureau; JAZZ: Honoraria, Speakers Bureau. Gupta:Roche: Other: Participation on a Data Safety or Advisory board; BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria; Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board; Novartis: Consultancy, Honoraria; AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy. Al-Ali:Sierra Oncology: Other: Medical Writing; Incyte, Deutsche Leukämie und Lymphom Stiftung, East German Study Group for Hematology and Oncology: Research Funding; BMS: Consultancy, Honoraria, Other: Support for attending meetings and/or travel, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel; AOP: Consultancy, Other: Support for attending meetings and/or travel; Abbvie, BluePrint, Takeda, Pfizer: Consultancy; Kartos: Membership on an entity's Board of Directors or advisory committees. Kuykendall:Pharmaessentia: Consultancy, Honoraria, Speakers Bureau; Imago Biosciences: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Blueprint: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; GSK - Sierra Oncology: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Prelude Pharmaceuticals: Other: Research Support; BMS: Consultancy, Honoraria, Other: Research Support, Speakers Bureau; Morphosys: Other: Research Support; Protagonist: Other: Research Support; CTI Biopharma: Consultancy, Honoraria, Speakers Bureau. Iurlo:Novartis, BMS, Celgene, Incyte, Pfizer: Honoraria. Kawashima:Sierra Oncology: Current Employment. Donahue:Sierra Oncology: Current Employment. Strouse:Sierra Oncology: Current Employment. Mesa:AOP: Consultancy; Promedior: Research Funding; Genotech: Research Funding; Samus: Consultancy, Research Funding; AbbVie: Research Funding; LaJolla Pharmaceutical: Consultancy; Sierra Oncology: Consultancy, Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; CTI: Research Funding; Roche: Consultancy; Celgene: Research Funding; Incyte: Consultancy, Research Funding; Blueprint: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Geron: Consultancy; Gilead: Research Funding; Imago: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal